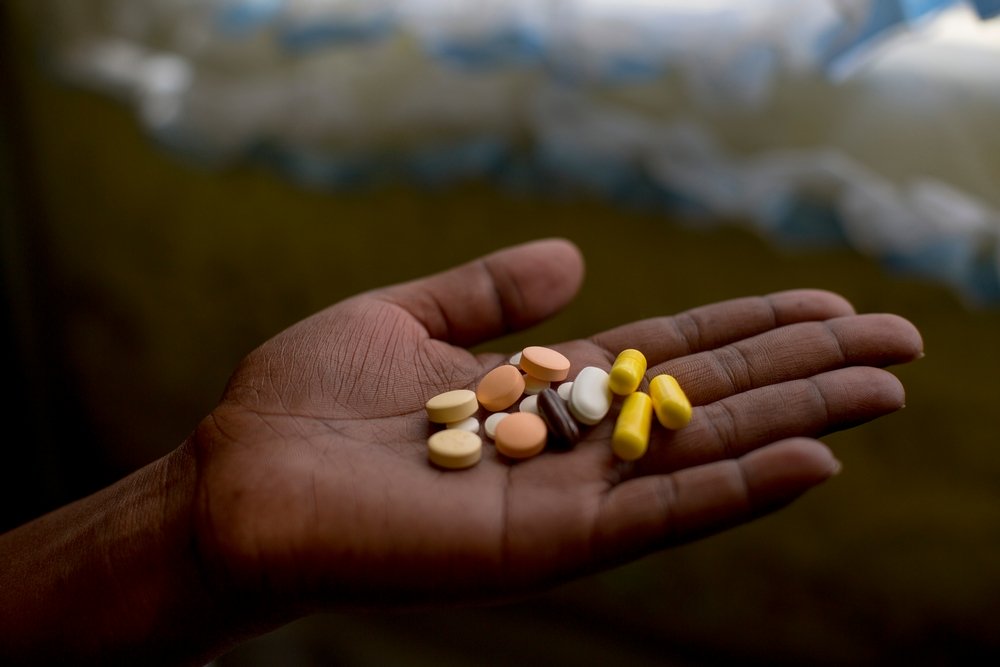
On 24 February, the Japanese pharmaceutical manufacturer Otsuka, announced a staggeringly high price tag of US$1,700 (R26,000) per treatment course for delamanid, one of only two new treatments in the world to be developed for drug-resistant tuberculosis (DR-TB) in the past 50 years.
What does this mean for South Africa?
South Africa, which has one of the highest global burdens of DR-TB with 18,000 cases diagnosed in 2014, is in fact one of the sites for Otsuka’s clinical trials on delamanid.
At least 7,000 people with DR-TB a year could benefit from this drug in South Africa, according to WHO guidance for using delamanid. Delamanid is particularly important for South Africa, as it can be included in DR-TB treatment for people co-infected with HIV.
Yet, while the South African Department of Health has high hopes of using delamanid as part of its ambitious DR-TB programme, yesterday’s news raises alarming questions as to how the country will access or afford this drug.
Despite having regulatory approval for delamanid in Europe for two years, Otsuka yet has still not yet filed for registration of delamanid in South Africa. To date, delamanid is only registered in four countries (Germany, Japan, South Korea, and the United Kingdom).
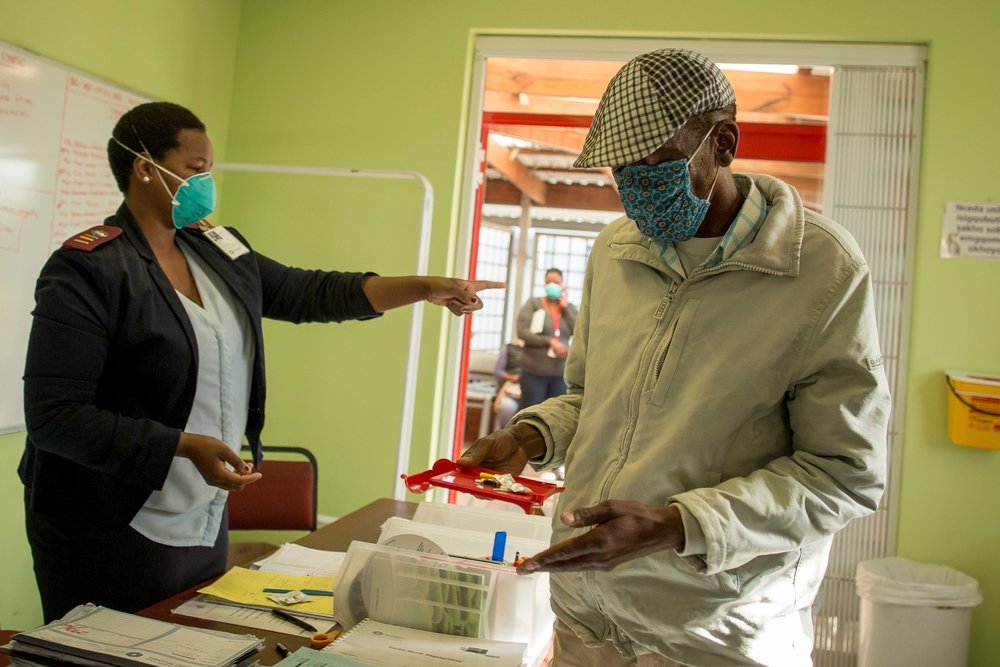
MSF recently gained access from Otsuka to a limited number of delamanid treatment courses for use in South Africa, and in the past three months, has started five DR-TB patients in Khayelitsha on regimens which include the drug. Ten other patients in Khayelitsha have also been identified as eligible for delamanid, and will start treatment once the drugs arrive in the country.
“MSF is treating some of the lucky few with DR-TB to have received delamanid in South Africa, and globally,” said MSF DR-TB doctor, Jennifer Hughes, “but the reality is that many DR-TB patients in South Africa could benefit now from having this drug in their treatment regimen, and simply won’t have access.”
MSF has been involved in tuberculosis (TB) care for 30 years, and in treating multidrug-resistant TB since 1999. MSF is now one of the largest NGO treatment providers for drug-resistant TB. In 2014, the organisation treated over 23,000 patients with TB, including 1,800 patients with drug-resistant TB.
MSF aims to scale up the use of delamanid in its programs, and in December 2015 accepted a donation of delamanid for use in MSF programmes and endTB partnership projects. DR-TB drug regimen prices are from the forthcoming MSF publication, DR-TB Drugs Under the Microscope, 4th Edition, to be published in March 2016.
Find out more about MSF in South Africa