One year since India and South Africa put forward the “TRIPS Waiver” proposal at the World Trade Organization (WTO), Doctors Without Borders (MSF) condemned the unrelenting opposition to this landmark initiative from a small group of WTO members, including the EU, UK, Norway and Switzerland.
First introduced on 2 October 2020, the TRIPS Waiver, which now has the support of over 100 nations, would waive patents and other intellectual property (IP) rights on urgently needed COVID-19 vaccines, treatments, tests and other health tools for the duration of the pandemic and pave the way for many countries to increase production and supply of these lifesaving medical tools.
If most of the world, including many countries where MSF works, continues to have only minimal access to COVID-19 vaccines, treatments and tests, this pandemic will continue, new and more transmissible variants may develop, healthcare systems will buckle, and more people will suffer and die.Dr Sharmila Shetty, Vaccines Medical Advisor, MSF Access Campaign
Dr Sharmila Shetty, Vaccines Medical Advisor, MSF Access Campaign
“In the one year that has passed since India and South Africa first tabled the landmark TRIPS Waiver in an effort to increase people’s access to COVID-19 medical tools, tragically over 3.6 million people have died from COVID-19.
“The world lost an entire year during which countless lives could have been saved and sustainable global access to COVID-19 medical tools equitably increased. A handful of governments have chosen to obstinately block the TRIPS Waiver from the beginning, and these are the same governments that have stockpiled vaccines and therapeutics to protect their own populations instead of sharing.
“If most of the world, including many countries where MSF works, continues to have only minimal access to COVID-19 vaccines, treatments and tests, this pandemic will continue, new and more transmissible variants may develop, healthcare systems will buckle, and more people will suffer and die.
“The EU, UK, Norway and Switzerland must stop obstructing the TRIPS Waiver and support the adoption of a groundbreaking effort that could increase access to COVID-19 medical tools for everyone, everywhere.”
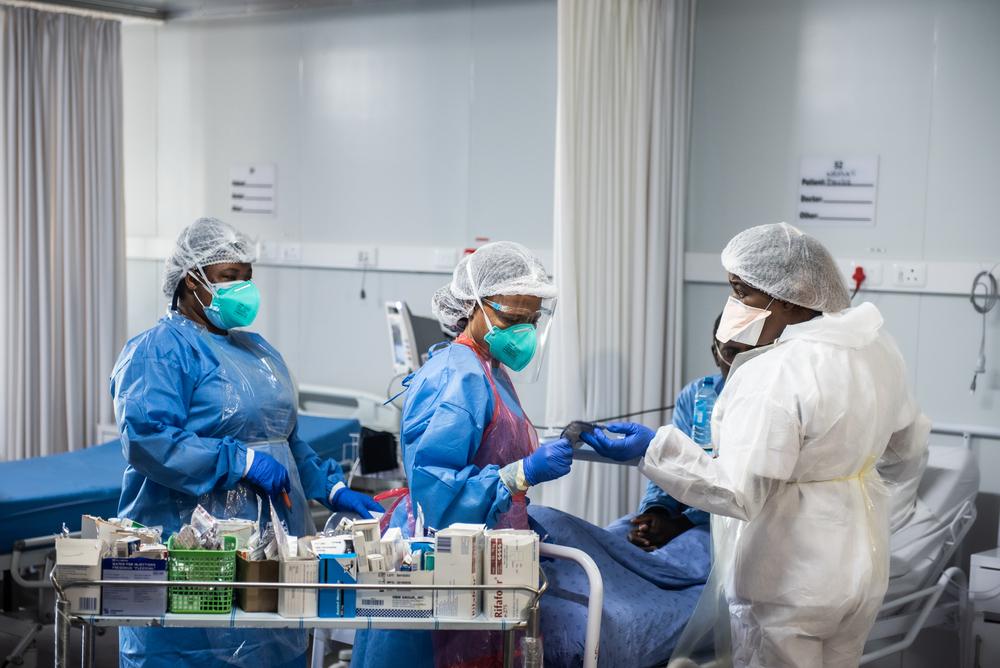
Our medical teams share their experiences and what they've witnessed during the pandemic. The urgency of their stories is what drives us to call for change to ensure equitable access to all COVID-19 medical tools.
In addition to supporting the TRIPS Waiver proposal, MSF calls on governments to use all legal and policy tools to facilitate uninterrupted production and diversity in the supply of COVID-19 medical tools, including the full use of existing TRIPS flexibilities for public health safeguarding. MSF also urges all governments with sufficient COVID-19 vaccine doses to immediately redistribute excess doses to the COVAX Facility. MSF urges the US and German governments to pressure Pfizer-BioNTech and Moderna to share mRNA vaccine technology and know-how with manufacturers in low- and middle-income countries, and for all governments to support the WHO COVID-19 mRNA Vaccine Technology Transfer Hub with financial and political support.

3 reasons why we need a Waiver on monopolies of ALL COVID-19 medical tools
There are no ‘silver bullets’ when it comes to COVID-19: while vaccines play a critical role in controlling the virus, they are just one of the many tools we need to beat this pandemic.
In addition to vaccines, the world urgently needs access to newer therapeutics and diagnostics to reduce the number of hospitalisations and deaths from COVID-19. With the slower and lower rate of vaccination in low- and middle-income countries due to access barriers, there is an increased risk of transmission and variant spread, both locally and globally.
Simultaneously, health systems are being pushed to their limits by ongoing and new waves of the virus around the world, once again highlighting the need to improve access to diagnosis and treatment of people with COVID-19 and to respond to the pandemic with a comprehensive and multifaceted approach.
A few treatments have been recommended by the World Health Organization (WHO) for the treatment of COVID-19, and pharma monopolies are already proving to be a barrier to accessing them.
Casirivimab and imdevimab, recently recommended by WHO for COVID-19 treatment, belong to the class of drugs called monoclonal antibodies (mAbs), which have been on the market for decades for other diseases and are often priced extremely high. Regeneron and Roche (responsible for pricing outside the US) have pegged casirivimab/imdevimab at the high price of US$820 in India, $2,000 in Germany and $2,100 in the US.
Furthermore, although casirivimab and imdevimab were recommended only recently, Regeneron has already begun applying for patents on the drugs in at least 11 low- and middle-income countries.
Independent developers of more affordable ‘biosimilar’ versions of mAbs often face patent barriers and regulatory challenges to introducing their products quickly to increase supply—something that is desperately needed as COVID-19 continues to rage across the globe. This has made production and supply of more affordable mAbs historically difficult, and it continues to undermine COVID-19 treatment access.
We urgently need to increase global supply of COVID-19 medicines. To do that, more manufacturers must be able to produce them through the waiving of intellectual property rights, and sharing of technology and know-how by the pharma industry.
Like casirivimab and imdevimab, tocilizumab and sarilumab are mAbs recommended by the WHO for COVID-19. Produced by pharma corporations Roche and Regeneron respectively, tocilizumab and sarilumab are recommended for the treatment of critically and severely ill COVID-19 patients and can potentially reduce reliance on medical ventilators and oxygen – both of which are in short supply in many of the settings where MSF works.
However, due to high prices and limited supply, these new therapeutics simply aren’t reaching everyone who needs them, as witnessed by MSF staff around the world.
“Over the last few months, we have helplessly witnessed people in South Asia scrambling to get hold of tocilizumab for patients with severe forms of COVID-19.”- Leena Menghaney, Global IP advisor for MSF Access Campaign.