- In the wake of postponement of WTO’s Ministerial, MSF underscores the urgency of adopting the TRIPS Waiver for people’s unhindered access to COVID-19 medical tools
- Doctors Without Borders and supporters urge high-income countries blocking the landmark TRIPS Waiver to put proclaimed global solidarity into practice and support the Waiver now
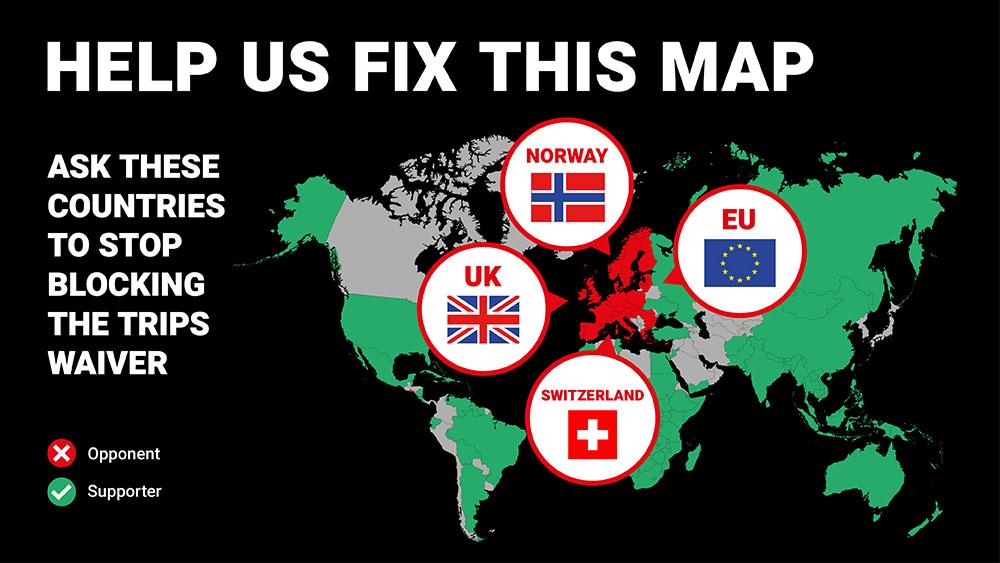
As the World Trade Organization (WTO) TRIPS Council meets today, after the indefinite postponement of the 12th Ministerial Conference due to the emergence of the highly transmissible strain of the COVID-19 virus along with changes in border rules for Switzerland, Doctors Without Borders (MSF) again deplored the dogmatic opposition to the landmark TRIPS Waiver proposal to temporarily waive intellectual property (IP) rights on COVID-19 medical products by a group of high-income countries including the European Union (EU), the United Kingdom and Switzerland.
With the COVID-19 pandemic showing no signs of abating, including in regions with unfettered access to COVID-19 medical tools and high vaccination rates, as in Europe, it is clear that prioritizing access to COVID-19 medical tools, including tests, treatments and vaccines for everyone, everywhere is essential.
“Recent emergence of another new, more transmissible variant is a telling example of how this virus continues to mutate particularly in the absence of equitable access to the right COVID-19 medical tools to deal with it,” said Candice Sehoma, South Africa Advocacy Officer, MSF Access Campaign. “With millions of lives at stake, the world can’t afford to waste any more time.

We call on countries opposing and diluting this waiver to today halt the stalling tactics and take urgent measures to adopt a comprehensive waiver for facilitating more diversified and broader production and supply of COVID-19 vaccines, therapeutics and diagnostics and other health technologies. The waiver is needed now more than ever.”
In the 14 months since India and South Africa first proposed the landmark TRIPS Waiver proposal in an effort to increase people’s access to COVID-19 medical tools, over 4 million people have lost their lives to COVID-19, with over 5 million lives lost since the start of the pandemic overall.
In response, over 100 nations have come together to support the TRIPS Waiver, showing that more than half the world’s governments consider the adoption and implementation of this proposal to be an effective tool against COVID-19. However, due to opposition from a group of high-income countries that are currently burying this global solidarity, negotiations on the TRIPS Waiver continue to move at a glacial pace. With the COVID-19 pandemic showing no signs of abating, this is unacceptable.
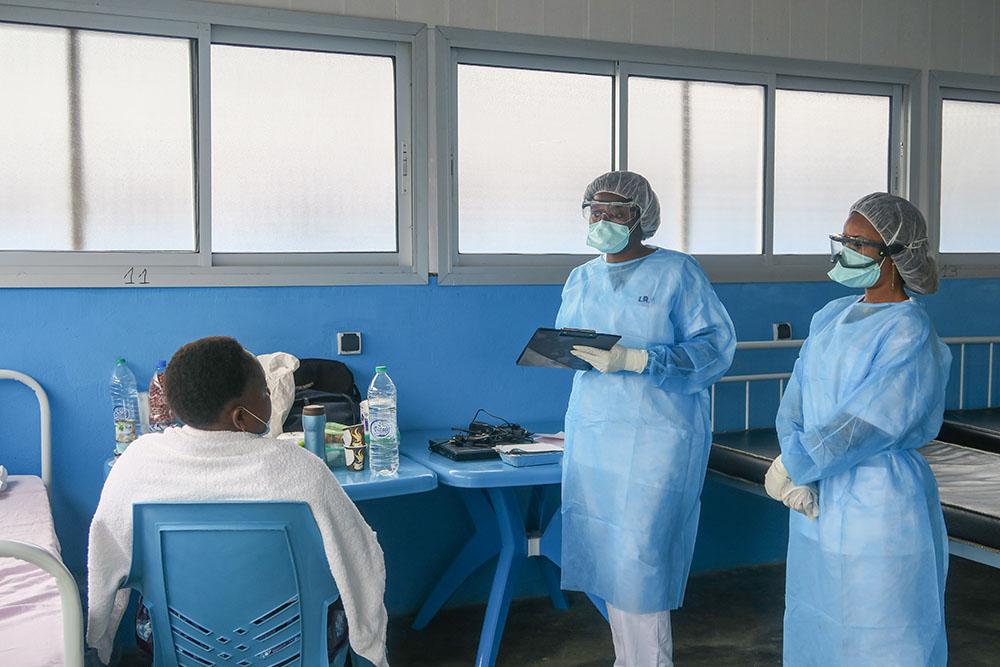
“Every day, we are witnessing a desperate need for COVID-19 medical tools in the places we work,” said Reveka Papadopoulou, President of MSF’s Operational Centre in Geneva. “Given the severely limited access to the COVID-19 drugs, diagnostics and vaccines needed to save lives, it’s truly demoralizing that some governments are opposing an initiative like the TRIPS Waiver which could have such a positive impact on how low- and middle-income countries are able to tackle this pandemic.”
As the world continues to experience severe inequity in access to COVID-19 vaccines, access to new treatments and tests to reduce the number of deaths remains crucial, yet similarly challenging. These access challenges are made worse because pharmaceutical corporations provide only a limited supply of medical tools to low- and middle-income countries whilst holding key patents and other IPs that can block generic production.

Every day, we are witnessing a desperate need for COVID-19 medical tools in the places we workReveka Papadopoulou, President of MSF’s Operational Centre in Geneva
New and potentially promising COVID-19 oral antiviral treatments, such as molnupiravir supplied by the pharmaceutical company Merck, face numerous access challenges. Despite Merck signing voluntary licenses with eight Indian generic companies and the Medicines Patent Pool (MPP), these agreements limit the countries where licensees can supply the drug, and the license with MPP contains a harmful clause that could undermine licensees’ legal rights to challenge molnupiravir patents. These limitations may create uncertainty, hindering the ability of other manufacturers in low- and middle-income countries to independently produce and supply generic molnupiravir.
Dependency on the pharmaceutical industry’s voluntary actions is often not a sufficient or sustainable way to increase global production and supply of lifesaving COVID-19 medical tools, highlighting the necessity of the TRIPS Waiver. And, while countries should take immediate actions to use all legal options that already exist in global trade rules, many of these may come with limitations in a pandemic context.
The TRIPS Waiver, if adopted, would provide an expedited option for governments to remove legal IP barriers on COVID-19 medical tools in the pandemic to facilitate increased and diversified production and supply, moving away from the overall dependence on corporate voluntary actions and towards autonomous production in low- and middle-income countries.
“Instead of supporting the TRIPS Waiver in actual solidarity, the EU and others have made attempt after attempt to erode this proposal to protect pharma companies’ interests at the expense of global access. With low- and middle-income countries demanding an opportunity to produce the medical tools needed to protect their people, this opposition to the TRIPS Waiver is utterly indefensible.
In order to be most effective, MSF has clearly outlined that the final text of the TRIPS Waiver must ensure the scope of the Waiver goes beyond vaccines and applies to all essential medical technologies including tests and treatments; that any and all IP and their enforcement are lifted; and that the duration of the Waiver lasts for at least 5 years in order to allow for the manufacturing and supply of COVID-19 medical tools and their materials and components to be prepared, scaled up, diversified and sustained.



