Doctors Without Borders (MSF) are pleased that, having shared the results of TB PRACTECAL, our clinical trial that found a new, safer and more effective six-month oral treatment regimen for multidrug-resistant tuberculosis (MDR-TB), the World Health Organization (WHO) has announced it will update global guidance on treatment.
Following the results of MSF’s trial the WHO are now recommending the programmatic use of the 6-month BPaLM regimen – comprising bedaquiline, pretomanid, linezolid (600 mg) and moxifloxacin in MDR-TB patients in place of longer, existing regimens. WHO is also recommending another shorter treatment; the BPaL combination in patients with increased drug resistance as both regimens showed high treatment success.
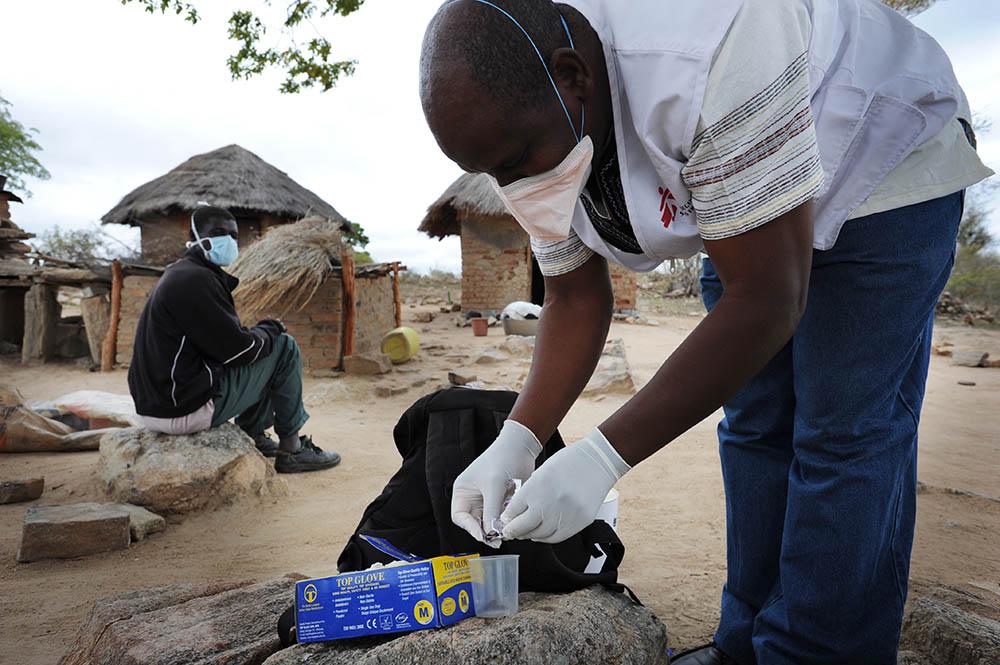
Launched in 2017, TB-PRACTECAL is the first-ever multi-country, randomised, controlled clinical trial to report on the efficacy and safety of a six-month, all-oral regimen for MDR-TB. The trial enrolled 552 patients overall in seven sites across Belarus, South Africa and Uzbekistan. The phase II/III clinical trial found that the new shorter BPaLM treatment regimen was very effective against rifampicin-resistant TB. 89 per cent of patients in the BPaLM group were cured, compared to 52 per cent in the standard of care group.
“[The shorter treatment] would mean a lot as I think when you are on treatment, some parts of your life feel like they are put on hold,” says Awande Ndlovu who was enrolled in the trial at the THINK Hillcrest Clinical Trial Unit in South Africa. “Before [the trial] gave me hope, I couldn’t even see the slightest glimpse of recovering from MDR-TB.”
While this new regimen provides new hope for the 500,000 people who fall sick each year with MDR-TB, the current lowest global price for a 6-month treatment course of BPaLM is US$800. This is still too high and could slow the uptake of the BPaLM regimen in high TB burden countries. Pretomanid—which was developed by TB Alliance and Viatris (previously Mylan) with the help of public funding—is priced at $336 for six months of treatment.
Before the trial gave me hope, I couldn’t even see the slightest glimpse of recovering from MDR-TB.Awande Ndlovu

“The groundbreaking TB PRACTECAL results found a six-month regimen of bedaquiline, pretomanid, linezolid and moxifloxacin (BPaLM) was more effective and safer than the standard of care for people affected with rifampicin-resistant forms of TB, but we will only see meaningful changes if treatment is affordable. Given the significant public funds that helped to pay for the development of both pretomanid and bedaquiline, these drugs should be affordable and accessible for everyone who needs them. We call on the TB Alliance, Viatris, and Johnson & Johnson to bring down the price of pretomanid and bedaquiline to ensure that the price of a complete MDR-TB treatment course is no more than $500 per person. Price should never be a barrier to accessing life-saving treatment.” says Christophe Perrin, TB advocacy pharmacist with MSF’s Access Campaign.
“When we embarked on this journey nine years ago, patients with MDR-TB around the world were facing lengthy, ineffective and gruelling treatment that disrupted their lives. Patients were telling us how hard it was to adhere to treatment, but little progress was being made to find kinder treatments because diseases most prevalent in low and middle-income countries don’t attract investment. So, we were compelled to pursue new treatment options ourselves. These results will give patients, their families and healthcare workers worldwide, hope for the future of MDR-TB treatment. We welcome the WHO’s decision to update the treatment guidelines. Now it is essential that national TB programs, ministries of health, and other key stakeholders ensure that this treatment is available to people with MDR-TB as soon as possible.” says Bern-Thomas Nyang’wa, MSF Medical Director and Chief Investigator of the trial.