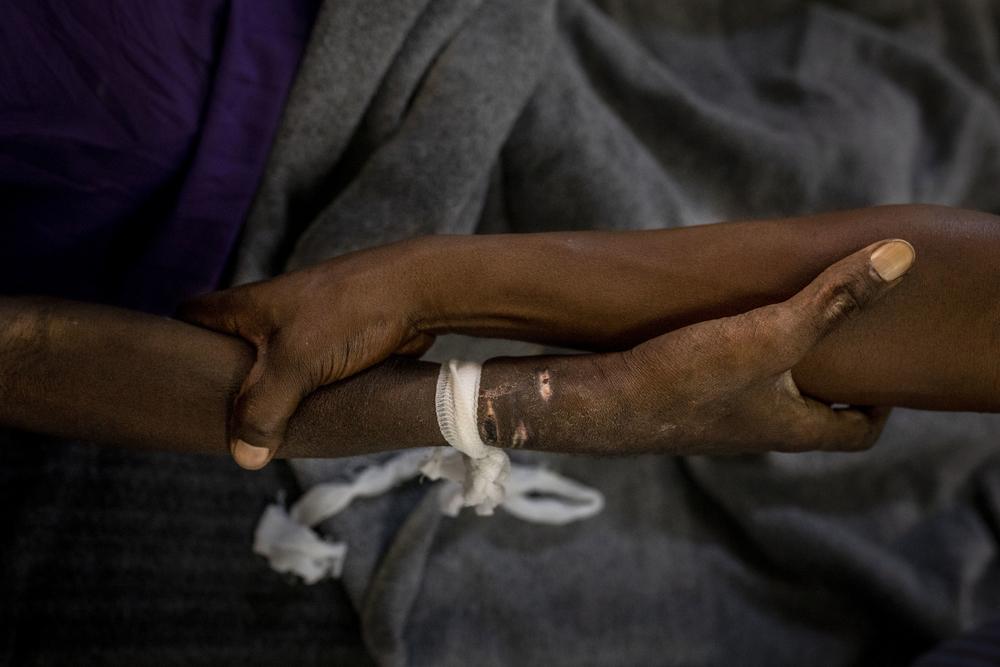
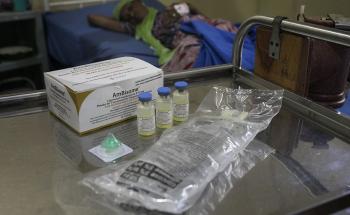
As negotiations continue on the declaration for the upcoming UN High-Level Meeting (HLM) on HIV/AIDS, Doctors Without Borders (MSF) urges all member states to recognise and retain the full rights and use of internationally agreed public health safeguards enshrined in the Agreement on Trade-related Intellectual Property Rights (TRIPs) for access to affordable generic antiretroviral (ARV) drugs and other medicines for HIV.
According to informed sources following the text negotiations of the UN HLM Declaration, several important paragraphs in the zero draft have become controversial, with some powerful countries trying to water down or delete texts addressing intellectual property (IP) and access to HIV medicines. This is troublesome in light of the fact that people living with HIV/AIDS still struggle to secure access to lifesaving treatment as a result of pharmaceutical monopolies. Some of the same countries also remain reluctant to back an important global waiver proposal on IP for COVID-19 medical tools.
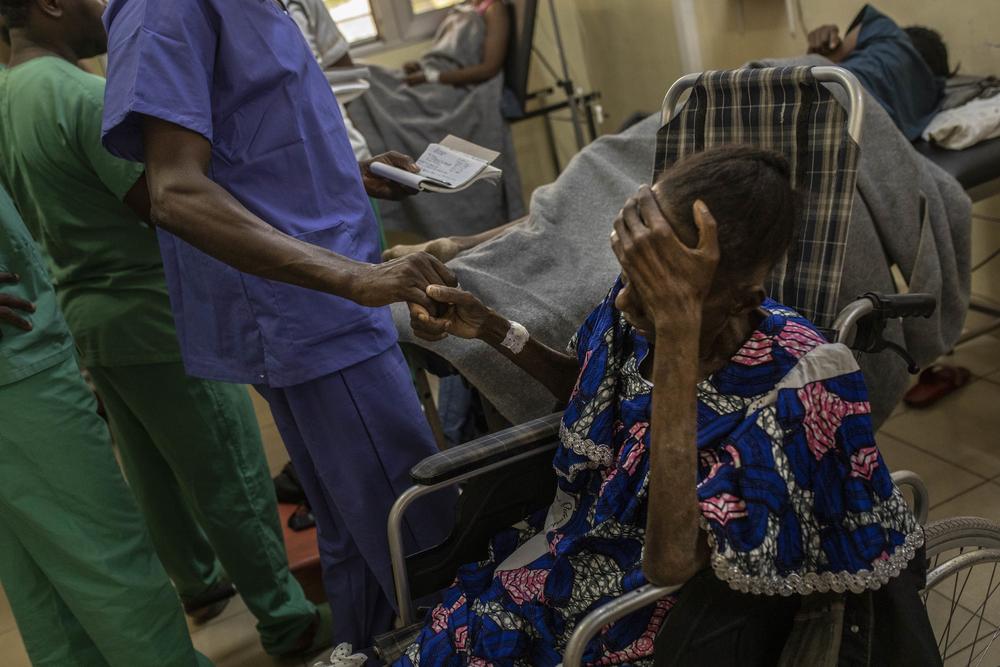
Among the controversial paragraphs under negotiation, developing countries have strongly demanded the inclusion of text supporting the full use of TRIPS flexibilities, safeguarding against harmful IP provisions in free trade agreements, and exempting countries from international IP obligations on medicines, diagnostics and other health technologies needed to prevent, diagnose and treat HIV and its co-infections and co-morbidities.
After two decades since the first generation of ARVs became available, we still face the reality of people living with HIV dying without access to affordable ARVs and treatments for opportunistic infections. Similarly, people co-infected with hepatitis C lack access to direct-acting antiviral (DAA) drugs. IP barriers such as patents and data exclusivity combined with restricted voluntary licensing terms and conditions exclude many middle-income countries from the benefits of generic drug competition.
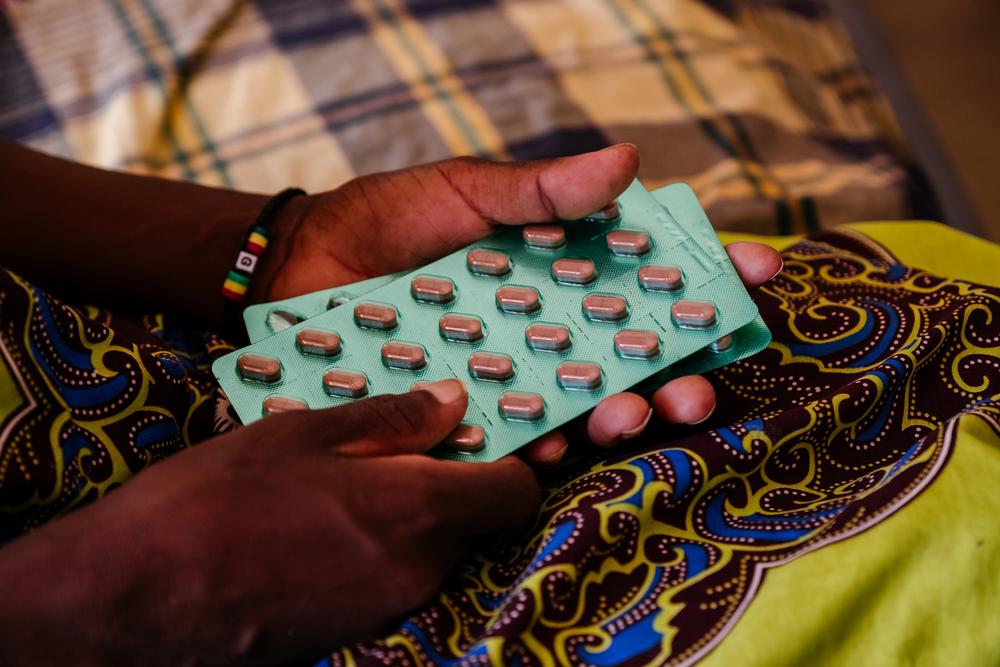
Newer generations of HIV treatments and drugs for pre-exposure prophylaxis (PrEP), such as long-acting injections, remain under patent monopolies, especially in middle-income countries where there is significant manufacturing capacity. Patient groups and treatment providers continue to spend an enormous amount of time and resources to challenge unmerited patents on medicines for drug-resistant TB, and DAAs for hepatitis C, delaying access to affordable generic medicines in several middle-income countries with a high burden of HIV.
It is critical that leaders remember this declaration won’t just live on paper; it will have real-world consequences for millions of people living with HIV.